HOW YOU CAN CONTRIBUTE
HOME
Obtaining research permits
Priority areas to visit
Species to look for
Collecting methods and study sites
Possible student projects
The diversity of species and habitats in Ecuador, and the relative ease of sampling of butterflies, provide ideal material for studying tropical diversity, biogeography, insect ecology and evolution. Even those with no entomological experience can, with sufficient motivation and enthusiasm, make valuable contributions to our knowledge of Ecuadorian butterfly biology and conservation, and broader natural processes.
OBTAINING RESEARCH PERMITS
HOME - -
TOP
Anyone doing biological research work in Ecuador will require a permit if that work involves collection of specimens. For most butterfly research, collection of specimens is necessary, if only to provide voucher specimens for the work conducted. Even the most common and distinctive "species" may contain two (or more) unrecognised sibling species (e.g., Willmott, Constantino & Hall, 2001); without specimens to permit identifications to be verified in future, data may become almost worthless. In any large scale diversity study collections are essential, as the taxonomy of a number of groups is still far from resolved and significant numbers of cryptic and undescribed species will certainly be recorded.
As of late 2019, a permit for country-wide research and collection of specimens may be obtained through the Ministerio del Ambiente with the backing of a sponsoring Ecuadorian institution, which will vouch for the value of your project and ensure that you comply with any requirements. Ordinarily, you will need to be affiliated with or employed by a research institution - university, museum or other appropriate body. Those without such affiliation should consider collaborating with someone who has such a position. Applications should be submitted 6 months prior to the start of field work, in Spanish. Broadly speaking, the following information will be required, although up-to-date, specific requirements should be clarified with the Ecuadorian sponsoring institution):
- Project description: detailed description of your intended project, including: project title, precise area of research, justification, objectives, methods, materials and equipment, specimen treatment/manipulation, methods for observation, methods for transporting specimens, justification for the quantity of specimens required, sampling sites, habitat management, expected results, analysis of abundance, diversity, frequency, density, endemism, species conservation status, environmental impacts, timetable of activities, technical equipment, project activities and project team, including principal investigators and collaborators. Note that every person who intends to collect specimens should be named on the permit unless accompanied by someone else named on the permit. Most importantly, the description should contain as precise information as possible on what taxa you intend to collect (e.g., Papilionidae, or Adelpha, or Heliconius erato) and how many specimens will be collected of each taxon and in total (an estimate). The project outline should also specify as precisely as possible where you intend to collect (e.g., Napo and Sucumbíos provinces, or Jatun Sacha Research Station, or Parque Nacional Yasuní); the permit will list all the provinces you are permitted to collect in and also any protected areas, as well as the taxa you are permitted to collect and numbers of specimens.
- Letter to the Curator of Entomology or appropriate contact at the sponsor institution asking for them to review your project and ask for the approval of the Head of the Institution for submission of the application to the Ministerio del Ambiente, and for them to issue a letter of support.
- Photocopies of passport photograph pages for all participants in the project.
- CVs for all principal participants in the project (translation of CVs into Spanish is recommended).
- Letter of approval from your own institution or one to which you are affiliated, vouching for you and approving your project.
- Letter to the Director of the Ministerio del Ambiente asking for their approval of your project and permit application.
- Letter of approval from the Ecuadorian sponsoring institution vouching for you and approving your project.
Once in the field, separate permits to move specimens out of the province where they were collected may be required, and specimens needed for export for identification and further research will require an export permit. Again, the sponsoring institution in Ecuador is the best source of information as to current requirements, and expectations regarding deposition of specimens and submission of data and reports.
For information about applying for permits through the INABIO contact:
Curator of Lepidoptera: Angélica Sofia Nogales Trujillo
Instituto Nacional de Biodiversidad (INABIO)
Calle Rumipamba 341 y Avenida de Los Shyris
Parque La Carolina, Quito
ECUADOR
Tel. 593 2 2449825 xt 111
PRIORITY AREAS TO VISIT
HOME - -
TOP
The map below shows almost all of the collection localities for butterflies in Ecuador known to us, and indicates that the butterfly faunas of a number of areas are still particularly poorly known. These areas are discussed below.
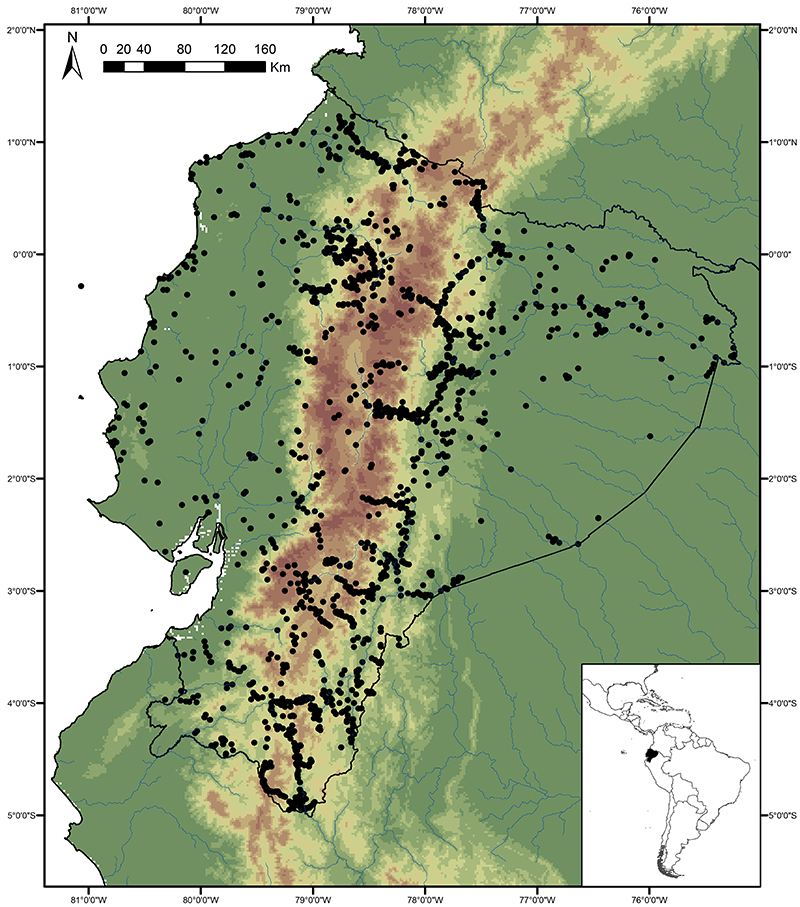
Far northeastern lowlands (Sucumbíos)
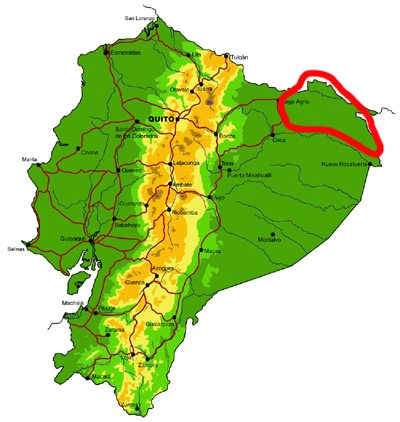
Knowledge and conservation: virtually nothing is known of the butterfly fauna north of the Río Aguarico and east of the town of Lago Agrio, with the exception of limited collecting visits by us and a handful of other researchers to Río Cuyabeno. Similarly, the adjacent region in Colombia is also poorly known for most groups, although the MNHN in Paris has a reasonably good collection of riodinids, and to a lesser extent lycaenids, from this area. Numerous new records of species and subspecies are expected for the country from this region. There has been extensive habitat loss due to oil exploitation since the 1970s, although part of the area is currently protected by the Reserva Producción Faunística Cuyabeno.
Access: Access to the region is by road from Lago Agrio to the town of Puerto Carmen del Putumayo, or by river, including Río Cuyabeno and Río Aguarico. It should be noted that this is a border region that may be dangerous for travel and up-to-date information should be obtained from local sources before travel. The wettest months are May-September and overall butterfly abundance probably peaks from August-October, although Riodinidae and Lycaenidae remain abundant throughout the subsequent drier months.
Far southeastern lowlands (Pastaza)
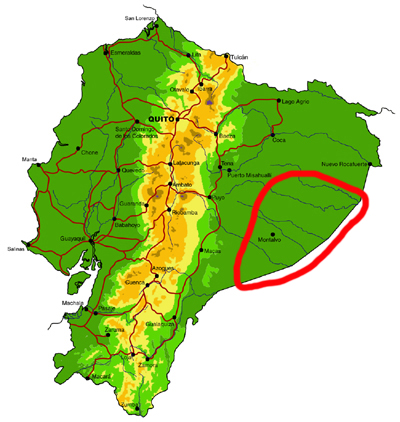
Knowledge and conservation: virtually nothing is known of the butterfly fauna south of the Río Cononaco and east of the town of Taisha. The adjacent region in Peru is also poorly known, but there is some material collected by Bassler in the AMNH in New York. Numerous new records of species and subspecies are expected for the country from this region. Large areas of undisturbed forest remain, but only a small section in the north of the area is protected, in the southern portion of the Parque Nacional Yasuní. The possibility of future habitat loss due to oil exploitation is high.
Access: Access to the region is by river, from the via Auca south of Coca (Río Cononaco), from the town of Morona on the Río Morona, or by light aircraft. TAME Amazonico and the military also fly from Shell to a number of airstrips, including Lorocachi on the Río Curaray (every few days) and other more obscure locations more infrequently. Most of these destinations require a high-level of self-sufficiency, given their remoteness. The best time of year to visit is probably similar to the northeastern lowlands (see above).
Southern montane
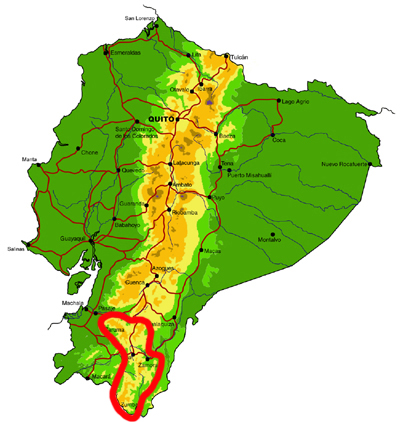
Knowledge and conservation: Southern Ecuador lies at the boundary between the northern and central Andean faunas, as well as being a region of endemism in its own right (with neighbouring northern Peru). Collections of butterflies have been made in this area for over a century, but the region is extremely complex and the vague label data on specimens of early collectors are often almost worthless. In addition, different valleys and elevations have different faunas, and not all have been sampled. Even in the Zamora valley, the most heavily collected areas, new species are still regularly being discovered. The Zumba region remains the most poorly known, with elevations ranging from 600-3600m. The Parque Nacional Podocarpus protects many of the species found in the region, though the Zumba region further south probably contains a significant number of additional species. Given the very high levels of endemism and habitat loss through centuries of human habitation, the conservation concern for this region is high.
Access: Access is by road from Loja to the towns of Zamora and Zumba, from where there are many side roads, often in very poor condition, and a 4-wheel drive vehicle is essential when exploring off the beaten track. There are also numerous long trails connecting remote villages. Some areas right on the Peruvian border may still contain land-mines from the historical conflict between Ecuador and Peru, so advice should be sought from the military or local villagers before venturing off road. This is generally a very wet area and the best times of year to visit are probably during the sunnier months of October-December and the wet-dry season transitional months of April-July.
Central and south western lowlands
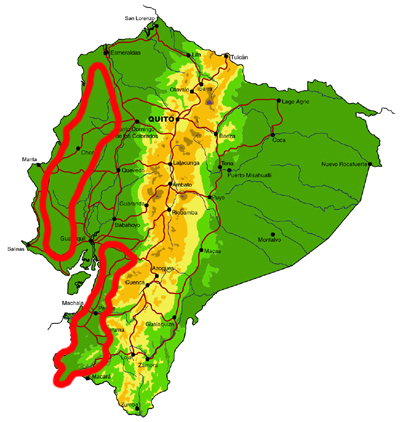
Knowledge and conservation: Ecuador’s lowland forests in the central and south west are the most highly threatened in the country, with less than 5% of original forest cover remaining. Despite historical accessibility, the area remains surprisingly poorly known, and new records of species otherwise known only from dry forests in Central America are still being made. In addition, endemism is high, with a number of species restricted to this region and northern Peru. Pockets of forest are protected in Parque Nacional Machalilla, Reserva Biológica Bilsa, Reserva Ecológica Mache-Chindul and a number of smaller, sometimes private, reserves, but protection of remaining habitat in the south is particularly poor.
Access: Roads and tracks traverse the entire region, though a 4-wheel drive vehicle is essential. The best time of year to visit appears to be April-July towards the end of the wet season.
Cordillera de Cutucú, Cóndor and Volcán Sumaco
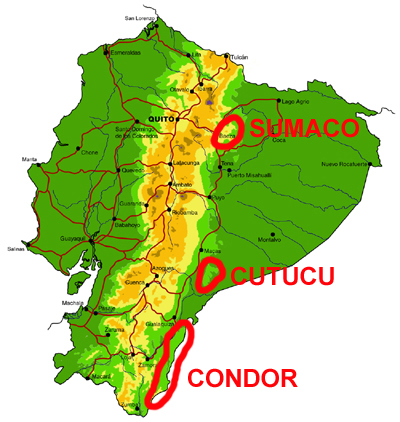
Knowledge and conservation: Relatively little is known of the butterfly faunas of these three isolated ranges lying east of the main Andean cordillera, with the exception of limited collections of butterflies in the Peruvian Cordillera del Cóndor by Lamas and colleagues, and the Cambridge University Lepidoptera Expedition to Ecuador in 2010 (e.g., Radford and Willmott, 2013). We know of only two collections made in the Cordillera de Cutucú, by Martin Cooper in the 1980s (east of Macas) and by ourselves in 2003 (east of Tayuza). The latter visit produced several new records for the country and new subspecies. Nothing is known of the butterflies of the higher slopes of Volcán Sumaco (>1400m). The latter lies in the Parque Nacional Sumaco-Galeras and is well-protected by its remoteness, while the Cordilleras de Cutucú and much of the Cóndor are currently unprotected despite growing evidence of considerable endemism.
Access: The Cordillera de Cutucú can apparently be accessed by several trails crossing the range, including one opposite Macas to the village of Mangosisca, which may or may not still exist, and the trail marked on some maps from Logroño/Tayuza to Yaupi. The Cordillera del Cóndor can be accessed by various roads, usually leading to military bases. Areas of the Cordillera del Cóndor were the scenes of historical border disputes between Ecuador and Peru, and might still contain land-mines. Access to Volcán Sumaco is via a trail from the village of Huamaní on the road between Tena and Loreto. It is reportedly a 3-5 day trip to the summit at 3732m, and a guide, which can be found in Huamaní, is essential.
SPECIES TO LOOK FOR
HOME - -
TOP
There are two classes of expected species that are likely to occur in Ecuador but have not yet been recorded. The first of these includes species that have: 1) been recorded on either side of the country in habitats that are present in Ecuador (usually Colombia and Peru); 2) been recorded close to the Ecuadorian border (<50km) in habitat that continues into Ecuador (e.g. several species collected by Lamas et al. (1996) on the Río Napo at the Peru/Ecuador border); or 3) species that are represented by putative Ecuadorian specimens that may be mislabelled but are still plausible members of the Ecuadorian fauna. These species invariably are almost certain to occur in Ecuador and we are therefore including them in our checklist of the country’s butterflies. A list of all such species is included here. Over the years many have been upgraded to confirmed records as knowledge of the country’s fauna has improved.
The second class of expected species includes those that do not satisfy the above criteria, but nevetheless have a reasonable probability of occuring in Ecuador. Such species are typically rare and known only from a few localities. Most commonly these are species that are known from the Iquitos region of Peru or the Chocó region of western Colombia, but no closer to Ecuador. For example, a recent student expedition from Cambridge University, UK, found four large, distinctive nymphalid species in western Ecuador that were previously known only as far south as central western or northern Colombia. These species are not included in our Ecuador checklist, but a list of such species in the Riodinidae only is included here as species to look for.
Finally, species continue to be found whose presence could not be predicted from any of the above criteria, especially in the Riodinidae and Lycaenidae, as well as completely unknown, new species. The not infrequent discovery of species in this third category makes Ecuador such an exciting place to work.
We include this section on the website to help lepidopterists more readily identify specimens of particular interest, and hopefully to encourage them to contact us with any new information on the country’s fauna.
BUTTERFLY SPECIES EXPECTED BUT NOT YET RECORDED IN ECUADOR (excluding Hesperiidae)
ADDITIONAL SPECIES WHOSE PRESENCE IN ECUADOR IS LIKELY (RIODINIDAE ONLY)
COLLECTING METHODS AND STUDY SITES
HOME - -
TOP
Unless the project targets a specific biogeographic area or elevation, the first decision to be made is the general region - does it contain sufficient environmental variation / habitat variation / species richness for the project in question? If the general location is fairly flexible, presence of research stations may be a deciding factor. Another possibility is to be based at a eco-tourist lodge, though these tend to be expensive. Below is a selection of possible research locations, though many others exist (many reserves are listed at http://www.ecuaworld.com/discover/reservas.htm.
Research stations:
West Andes and lowlands:
Estación Biológica Lalo Lahoor (Manabí: 0-450m) (http://www.jatunsacha.org/ingles/home.htm)
Estación Biológica Bilsa (Manabí: 300-800m) (http://www.jatunsacha.org/ingles/home.htm)
Estación Biológica La Hesperia (Pichincha: 1100-2000m) (http://www.jatunsacha.org/ingles/home.htm)
Río Palenque Science Center (Los Rios: 130-188m) (http://www.fundacionwong.org)
Reserva Maquipucuna (Pichincha: 1300-1700m) (http://www.arches.uga.edu/~maqui)
East Andes and lowlands:
Lagunas de Cuyabeno, Río Cuyabeno (Sucumbios: 200m)
Estación Científica Yasuní (Napo: 250-350m) (http://www.puce.edu.ec/facultades/cnaturales/biologicas/yasuni/yasuni.htm)
Tiputini Biodiversity Station (Napo: 250-350m) (http://www.usfq.edu.ec/1TIPUTINI)
Estación Biológica Jatun Sacha (Napo: 400-500m) (http://www.jatunsacha.org/ingles/home.htm)
Parque Nacional Podocarpus (Río Bombuscaro) (Zamora-Chinchipe: 1000-1300m) (informative travel agent site: http://www.thebestofecuador.com/pnodo.htm)
Estación Científica San Francisco (Zamora-Chinchipe: 1500-1900m) (http://www.mtnforum.org/resources/library/ecsf98a.htm)
Yanayacu Biological Station (Napo: 2000-2500m) (http://www.yanayacu.org)
Parque Nacional Podocarpus (Cajanuma) (Loja: 2800-3000m) (informative travel agent site: http://www.thebestofecuador.com/pnodo.htm)
Estación Biológica Guandera (Carchi: 3100-3600m) (http://www.jatunsacha.org/ingles/home.htm)
Selected eco-tourist lodges:
Western lowlands:
Reserva de la Montaña Río Chuchuví (Esmeraldas: 700-900m) (http://www.chuchuvi.net)
Eastern lowlands:
La Selva Lodge, Río Napo (Sucumbíos: 200m) (http://www.laselvajunglelodge.com)
Sacha Lodge, Río Napo (Sucumbíos: 200m) (http://www.sachalodge.com)
Kapawi Lodge, Río Bobonaza (Pastaza: 200m) (http://www.kapawi.com)
Yuturi and Yarina Lodges, off Río Napo (Orellana: 200-300m) (http://www.yuturilodge.com)
If your project requires a range of elevations or regions to be sampled, you will probably have to find your own sample sites. Almost all land in Ecuador is owned by someone, even when it appears to be untouched forest. However, most land-owners, at least outside of the western lowlands, will agree to give you access to their land if you ask permission and explain your intentions. If the land-owner is not to be found in a nearby house, this is a fruitful place to find out where they might be. Existing trails, to houses, villages, fields, for hunting or for logging, provide the most convenient access. Such trails usually make use of "friendly" topographic features like ridge-tops and make the physical collection of specimens more straightforward and reduce the chance of becoming lost. However, the increase in light around trails, different plant composition, disturbance and other non-random features may severely bias results in some studies, and transects may have to be marked out. For simple inventories a variety of trails should be sought, since species may be specific to river-sides, ridge-tops, forest understorey, and other restricted micro-habitats.
Unless based at a single site, you will need to consider transport to field sites. Public transport is frequent and cheap in most areas, but some regions will require you to have your own vehicle. Hire cars are expensive (similar in cost to Europe and USA) and available only in Quito, Cuenca, Guayaquil and perhaps Loja. An alternative is to employ a driver and car, which may not cost much more. For many years we have worked with Ismael Aldas (Baños), who also has substantial knowledge of the Ecuadorian butterfly fauna and has contributed significantly to our fieldwork in the country. Ismael can be contacted at taisae2007@hotmail.com.
Collecting of adult butterflies
Hand-netting

La Punta, Esmeraldas, northwestern Ecuador
The most important feature of any net to be used in sampling tropical butterflies is the length of the net handle - 4m is a good minimum, although we use net handles up to 15m in length. Aluminium net poles that snap-lock or screw together (such as provided by BioQuip) are usually capable of reaching up to 8m or so. Other methods we have used to collect butterflies from the subcanopy include using a portable aluminium ladder and single rope ascension techniques. Canopy towers or cable cars, present at some lowland research stations and lodges, also provide easy access to the highest levels of the forest.
Trapping

Butterfly bait traps in action
Trapping is essential for any serious inventory of butterflies in the tropics. Traps should consist of rot-proof netting (e.g., nylon net curtain material) made into a cylinder and closed at the top, from which a base of plywood or stiff plastic board is suspended to hold the bait (click here for PDF diagram). The type of bait is critically important in influencing abundance, diversity and identity of species captured. There are two principle classes of baits: of plant origin or of animal origin. The former consists of rotting fruits and/or mixtures of sugar, molasses, alcoholic beverages and various other sweet substances. These baits mainly attract both sexes of Satyrinae and certain nymphalid subfamilies, particularly Brassolinae, Morphinae and some Biblidinae. The latter class of baits consists of rotting carrion (fish is especially effective and easy to obtain), faeces, urine, and numerous other unpleasant substances. Fish bait should be made by dicing whole fresh fish (marine or freshwater, discarding head and tail) into 1cm cubes and placing these to fill half of a plastic jar (chemical jars are ideal). Water should be added to fill the space between the flesh and the jar sealed. The lid should be released at least twice daily during fermentation, which takes about 3-4 days in the lowlands and a week in the highlands. These baits attract almost exclusively males (with the exception of Lycaenidae) of all nymphalid subfamilies, as well as Riodinidae and Lycaenidae. Traps can be deployed in a standardised manner for quantitative sampling (see below), or set near the edges of small light-gaps to maximise abundance and diversity of butterflies captured. Traps should be patrolled throughout the day since many species rest or feed only on the outside of the netting. For the truly die-hard collector, traps can be made even more attractive by the spreading of fish bait on the outside of the netting.
Specimen storage Specimens for museum collections can be stored in the field in paper stamp envelopes. Specimens should be kept in a wooden entomological box or open plastic box until dried. If kept in an airtight box they will quickly develop mould, although camphor crystals help in reducing this problem. If attack from other insects, such as ants, is a problem, boxes should be placed on top of an upturned cup in a dish of water. If specimens are desired for molecular analysis, they should either be rapidly dried using silica gel, or the wings removed, stored in a paper stamp envelope, and the entire body immersed in a vial of industrial or medicinal alcohol (ethanol, available in Ecuador in pharmacies or hardware stores). Both envelope and vial should receive the same unique voucher number. Other details on collecting specimens for molecular study can be found in: Carter, D. J., A. F. Vogler, and R. I. Vane-Wright, 1997, Notes on basic collecting techniques for morphological and molecular studies of Lepidoptera, Metamorphosis, 8(3): 99-106.
Quantitative sampling of adult butterflies
Walk/count For quantitative estimates of butterfly diversity and abundance, one widely used technique involves walking and counting individuals of species. Trails or transects are marked out and then walked at a constant pace by 1-2 observers. One observer captures or records all individuals within an imaginary box 5m to either side and 5m in front of the observer. All individuals seen are recorded, with additional data such as time of day, flight height and behaviour, depending on the study.
Trapping Traps have been used to quantify flight heights, as well as compare abundance and diversity between different habitats. Abundance, diversity and identity of captured species depend on the type of bait used, and it is usually wise to try using different classes of baits to avoid spurious results. Traps provide a method of sampling that can be standardised between different observers, as well as permit specimens to be identified in the hand without the need for collecting by net.
Mark/Release/Recapture (MRR) When specimens are not required for identification, individuals collected by net or trap may be marked on the underside of the wings using a non-toxic permanent marker pen. Data from subsequent recaptures may be used to estimate butterfly population size, movement, age, and mortality rates.
Immature stages
Some studies may require collection of ecological information on immature stages, as well as specimens of these stages. Knowledge of immature stages is incomplete for virtually all neotropical butterflies, and non-existent for the vast majority.
Immature stages may be located by following adult females as they search for foodplants, then rearing any eggs or other stages located on the plant. Alternatively, potential foodplants may be methodically searched by turning leaves (many species rest under leaves), or by looking for distinctive leaf damage. Many Biblidinae and Charaxinae characteristically leave the leaf mid-rib intact while feeding, sometimes resting on this. In Adelpha a pile of frass is accumulated at the base of this vein. Many Hesperidae and some species from other groups (e.g., Memphis, Charaxinae) make leaf shelters from rolled, cut and/or sewn leaves, which are highly distinctive with experience. Another method is to collect eggs and attempt to rear them on probable host-plants, either by offering probable foodplants to captive females and hoping for oviposition, or by expressing an egg or eggs from the abdomen of captured adult females using gentle pressure of the thumb and forefinger on the side of the abdomen.
Records should be made of the species of plant on which any stages were found and or/fed in nature or captivity, and voucher specimens of the plant collected to verify identification. A description of the location of the plant, and the location on the plant of the immature stages, are also both important. Eggs and larvae may be reared in transparent plastic bags kept frequently (every 2-4 days) stocked with fresh foliage. Notes should be taken of the appearance of different instars and dates of moults, and head capsules, cast skins and empty pupal cases should all be preserved with the adult voucher specimen associated with the record. Where possible, specimens of each stage should be preserved, most conveniently in industrial or medicinal alcohol (ethanol), though other methods produce much better preserved specimens (e.g. see DeVries, P.J., 1997, The Butterflies of Costa Rica and their Natural History. Volume II. Riodinidae. Princeton: Princeton University Press). Without adult voucher specimens keyed via a unique identifying number to all associated data and specimens, the information may become worthless.
POSSIBLE STUDENT RESEARCH PROJECTS
HOME - -
TOP

Emma McLoughlin of Cambridge University
Expedition to northwest Ecuador, 2002
The following are a few simple to not so simple ecological research topics that could be performed by students with butterflies in Ecuador. There is also substantial potential for conservation projects involving butterflies, but these are best developed through discussion with specific conservation groups. A third possibility is to undertake basic faunistic inventories in hitherto unexplored regions. Such inventories provide the baseline data for conservation decisions, as well as raw data for biogeographic studies, and species new to science will almost certainly be discovered, as well as new records for the country. Poorly known areas of the country that would be worth visiting are listed here. We would be willing to provide advice to those who are genuinely likely to do research work on Ecuadorian butterflies (contact us). Asterisks before bibliographic references indicate relevance.
Species richness patterns and their measurement
Correlation between environmental gradients and species richness
Hypotheses: species richness is correlated with environmental variables, such as mean annual temperature, mean annual rainfall, mean monthly rainfall, solar radiation, elevation, rainfall variability, and potential evapotranspiration.
Method: trapping and/or hand netting to measure species richness and abundance at sites across an environmental gradient.
Suggested location: western lowlands, which range from c. 8m rain per year in far northwest to <1m per year in far southwest.
Background: Recent papers have suggested that ranges, and therefore species richness, are randomly distributed with respect to environmental gradients. Western Ecuador has a very steep environmental gradient that is also asymmetric with respect to the equator and the geographical limits of the Transandean region (Central America to W. Ecuador), providing a suitable location to test the hypothesis.
References:
**Brehm, G., J. Humeier, and K. Fiedler (2003). Beta diversity of geometrid moths (Lepidoptera: Geometridae) in an Andean montane rainforest. Diversity and Distributions, 9: 351-366.
**Lees, D. C., C. Kremen, and L. Andriamampianina (1999). A null model for species richness gradients: bounded range overlap of butterflies and other rainforest endemics in Madagascar. Biological Journal of the Linnean Society, 67: 529-584.
**Colwell, R. K., and G. C. Hurtt (1994). Nonbiological gradients in species richness and a spurious Rapoport effect. American Naturalist, 144(4): 570-595.
**Brown, J. H. (1984). On the relationship between abundance and distribution of species. American Naturalist, 124(2): 255-279.
Species richness in different habitats
Hypothesis: habitat influences species diversity (different habitats have predictably different diversities).
Method: trapping and/or hand-netting in different natural and disturbed habitats. Comparison of diversity measures. Faunal composition: is there a correlation between species that are quickly lost from primary habitats and endemism, local rarity, canopy vs. understorey life style, etc.?
Suggested location: forest habitats anywhere in country.
Background: this is a relatively well researched area, with most studies finding elevated species richness in slightly disturbed habitats, with lower richness of narrowly endemic species, but there is still significant variation in results. At least two projects have been done in western Ecuador lowland forest, where there is human disturbance. The effect of natural disturbances within forest (river banks, tree-falls) is currently unexplored.
References:
**Hamer, K. C., J. K. Hill, L. A. Lace, and A. M. Langan (1997). Ecological and biogeographical effects of forest disturbance on tropical butterflies of Sumba, Indonesia. Journal of Biogeography, 24: 67-75.
**Hill, J. K., K. C. Hamer, L. A. Lace, and W. M. T. Banham (1995). Effects of selective logging on tropical forest butterflies in Buru, Indonesia. Journal of Applied Ecology, 32(4): 754-760.
**DeVries, P. J., D. Murray, and R. Lande (1996). Species diversity in vertical, horizontal, and temporal dimensions of a fruit-feeding butterfly community in an Ecuadorian rainforest. Biological Journal of the Linnean Society, 62: 313-341.
Spitzer, K., V. Novotny, M. Tonner, and J. Leps (1993). Habitat preferences, distribution and seasonality of the butterflies (Lepidoptera, Papilionoidea) in a montane tropical forest, Vietnam. Journal of Biogeography, 20: 109-121.
DeVries, P. J., T. R. Walla, and H. F. Greeney. (1999). Species diversity in spatial and temporal dimensions of fruit-feeding butterflies from two Ecuadorian rainforests. Biological Journal of the Linnean Society, 68: 333-353.
Thomas, C. D. (1991). Habitat use and geographic ranges of butterflies from the wet lowlands of Costa Rica. Biological Conservation, 55(3): 269-281.
Thomas, C. D., and H. C. Mallorie (1985). Rarity, species richness and conservation: butterflies of the Atlas mountains in Morocco. Biological Conservation, 33: 95-117.
Distribution of butterfly species richness and endemism along elevational gradients
Hypothesis: species richness decreases monotonically with elevation, and species turnover is uniform.
Method: sampling of species abundance and richness along elevational transect to determine patterns of richness and species turnover; is there a uniform decrease in species richness with elevation, or a mid-elevation peak in richness? Are faunas elevationally zoned? Do zones of high species turnover correlate with high species richness? Do all taxonomic groups show similar patterns?
Suggested location: east Andean slopes: Napo region (Tena-Quito road via Baeza, 500-3000m); Sucumbíos region (Lumbaquí to Tulcán road, 500-3000m),
Background: Few studies have explored this pattern and there is still disagreement, with very few data in particular on changing abundance.
References:
**Brehm, G., D. Süssenbach, and K. Fiedler (2003). Unique elevational diversity patterns of geometrid moths in an Andean montane rainforest. Ecography, 26: 456-466.
**Grytnes, J. A., and O. R. Vetaas (2002). Species richness and altitude: a comparison between null models and interpolated plant species richness along the Himalayan altitudinal gradient, Nepal. American Naturalist, 159: 294-304.
**Holloway, J. D., G. S. Robinson, and K. R. Tuck (1990). Zonation of the Lepidoptera of northern Sulawesi. In: Knight, W. J., and J. D. Holloway (eds.), Insects and the Rain Forests of South East Asia (Wallacea). London: Royal Entomological Society of London. Pp. 153-166.
**McCoy, E. D. (1990). The distribution of insects along elevational gradients. Oikos, 58: 313-322.
**Hanski, I. (1983). Distributional ecology and abundance of dung and carrion-feeding beetles Scarabaeidae in tropical rain forests in Sarawak, Borneo. Acta Zoologica Fennica, 167: 1-45.
*Rahbek, C. (1997). The relationship among area, elevation, and regional species richness in neotropical birds. American Naturalist, 149(5): 875-902.
Terborgh, J. (1985). The role of ecotones in the distribution of Andean birds. Ecology, 66(4): 1237-1246.
Species loss within habitat "islands"
Hypothesis: fragmentation of forest into islands leads to loss of species as predicted by richness/area curves, with possible time lag.
Method: sample variety of forest islands; test for correlation between species lost most rapidly from small islands and dispersal ability, canopy vs. understorey lifestyle, range size etc.
Suggested location: forest fragments in southwest Ecuador; elfin forest islands in Andes.
Background: A huge body of literature on the subject, but no work done on butterflies (or many other groups) in either of the suggested regions, both of which are of high conservation concern.
References:
**MacArthur, R. H., and E. O. Wilson (1967). The Theory of Island Biogeography. New Jersey: Princeton University Press.
**Brooks, T. M., S. L. Pimm, and N. J. Collar (1997). Deforestation predicts the number of threatened birds in insular Southeast Asia. Conservation Biology, 11(2): 382-394.
**Brooks, T. M., S. L. Pimm, and J. O. Oyugi (1999). Time lag between deforestation and bird extinction in tropical forest fragments. Conservation Biology, 13(5): 1140-1150.
*Dennis, R. L. H., and T. G. Shreeve (1997). Diversity of butterflies on British islands: ecological influences underlying the roles of area, isolation and the size of the faunal source. Biological Journal of the Linnean Society, 60(2): 257-275.
Brown, K. S., Jr. (1991). Conservation of Neotropical environments: Insects as indicators. In: Collins, N. M., and J. A. Thomas (eds.), The Conservation of Insects and their Habitats. London: Academic Press. Pp. 349-404.
Effects of forest edges on butterfly fauna
Hypothesis: intermediate amounts of habitat disturbance create an increase in local diversity.
Method: conduct trapping and hand-netting across a forest edge transect; examine effect of edge on faunal composition of both canopy and understorey, and distance over which influence is exerted.
Suggested location: eastern lowland forest.
References:
**Murcia, C. (1995). Edge effects in fragmented forests: implications for conservation. Trends in Ecology and Evolution, 10(2): 58-62.
**Brown, K. S., Jr. (1991). Conservation of Neotropical environments: Insects as indicators. In: Collins, N. M., and J. A. Thomas (eds.), The Conservation of Insects and their Habitats. London: Academic Press. Pp. 349-404.
Brown, K. S., Jr. (1997). Diversity, disturbance and sustainable use of neotropical forests: insects as indicators for conservation monitoring. Journal of Insect Conservation, 1(1): 25-42.
Pinheiro, C. E. G., and V. C. Ortiz (1992). Communities of fruit-feeding butterflies along a vegetation gradient in central Brazil. Journal of Biogeography, 19: 505-511.
Lovejoy, T. E. et al. (1986). Edge and other effects of isolation on Amazon forest fragments. In: Soulé, M. E. (ed.), Conservation Biology: The Science of Scarcity and Diversity. Sunderland: Sinauer. Pp. 257-285.
Effect of sampling technique and location on diversity estimates
Hypothesis: different sampling techniques and locations yield similar estimates of relative diversity for different habitats.
Method: select a primary and a secondary forest site and sample a diversity of microhabitats in each (e.g., ridge-tops, hill-sides, river-sides, forest edges [natural and man-made], large and small light gaps, different heights above ground [trap position or length of net pole], in shade or sun) using: traps, baited with fruit or carrion baits, and hand-netting transects. Estimate diversity from species/abundance indices, and richness from species/abundance accumulation curves and species/effort accumulation curves.
Suggested location: eastern lowland forest.
Background: very little is known of the effects of sampling strategy on diversity estimates, yet associated errors may be larger than the differences that the sampling is designed to detect.
References:
**Sparrow, H. R., T. D. Sisk, P. R. Ehrlich, and D. D. Murphy (1994). Techniques and guidelines for monitoring neotropical butterflies. Conservation Biology, 8(3): 800-809.
**DeVries, P. J., D. Murray, and R. Lande (1997). Species diversity in vertical, horizontal, and temporal dimensions of a fruit-feeding butterfly community in an Ecuadorian rainforest. Biological Journal of the Linnean Society, 62: 313-341.
**Soberón M., J., & J. Llorente (1993). The use of species accumulation functions for the prediction of species richness. Conservation Biology, 7(3): 480-488.
*Pogue, M. G. (2000). Preliminary estimates of Lepidoptera diversity from specific sites in the Neotropics using complementarity and species richness estimators. Journal of the Lepidopterist's Society, 53(2): 65-71.
Butterflies as biodiversity indicator species
Hypothesis: patterns of butterfly species richness may be used to predict those of other taxonomic groups (e.g., birds, mammals, reptiles, plants).
Method: sample different natural habitats, or range of disturbed habitats, to obtain species diversity estimates, patterns of endemism, patterns of rarity (do rare species occur together?). Examine correlations with similar data for other groups.
Suggested location: depends on availability of data for other groups.
Background: idea of 'indicator groups' still debated. The scale may be highly significant (i.e. comparison of forest patches compared to comparison of biogeographic regions).
References:
**Ricketts, T. H., G. C. Daily, and P. R. Ehrlich (2002). Does butterfly diversity predict moth diversity? Testing a popular indicator taxon at local scales. Biological Conservation, 103: 361-370.
**Kerr, J. T., A. Sugar, and L. Packer (2000). Indicator taxa, rapid biodiversity assessment, and nestedness in an endangered ecosystem. Conservation Biology, 14: 1726-1734.
**McGeoch, M. A. (1998). The selection, testing and application of terrestrial insects as bioindicators. Biological Reviews, 73: 181-201.
**Lawton, J. H. et al. (1998). Biodiversity inventories, indicator taxa and effects of habitat modification in tropical forest. Nature, 391: 72-75.
**Prendergast, J. R., R. M. Quinn, J. H. Lawton, B. C. Eversham, and D. W. Gibbons (1993). Rare species, the coincidence of diversity hotspots and conservation strategies. Nature, 365: 335-337.
*Williams, P., D. Gibbons, C. Margules, A. Rebelo, C. Humphries, and R. Pressey (1996). A comparison of richness hotspots, rarity hotspots, and complementary areas for conserving diversity of British birds. Conservation Biology, 10(1): 155-174.
Prendergast, J. R., and B. C. Eversham (1997). Species richness co-variance in higher taxa: empirical tests of the biodiversity indicator concept. Ecography, 20: 210-216.
Brown, K. S., Jr. (1991). Conservation of neotropical environments: insects as indicators. In: N. M. Collins, and J. A. Thomas (eds.), The Conservation of Insects and their Habitats. London: Academic Press. Pp. 351-404.
Thomas, C. D., and H. C. Mallorie (1985). Rarity, species richness and conservation: butterflies of the Atlas mountains in Morocco. Biological Conservation, 33: 95-117.
Butterfly sub-groups as indicators of butterfly diversity
Hypothesis: some butterfly groups may be better predictors of total butterfly species richness and endemism than others.
Method: sample different habitats, or range of disturbed habitat, using hand-netting and trapping. Examine both logistical value of group (ease and speed of sampling, measured by rate at which species/effort curves approach asymptote) and ability as indicator taxon (correlation between group richness and total butterfly richness).
Suggested location: eastern lowlands and montane forest.
Background: idea of 'indicator groups' still debated. The scale may be highly significant (i.e. comparison of forest patches compared to comparison of biogeographic regions).
References:
**Beccaloni, G. W., and K. J. Gaston (1994). Predicting the species richness of Neotropical forest butterflies: Ithomiinae (Lepidoptera: Nymphalidae) as indicators. Biological Conservation, 71: 77-82.
Brown, K. S., Jr. (1991). Conservation of neotropical environments: insects as indicators. In: N. M. Collins, and J. A. Thomas (eds.), The Conservation of Insects and their Habitats. London: Academic Press. Pp. 351-404.
Courtship and mating
Mate-locating behaviour in forest butterflies
Hypothesis: perching at a particular site by males is an adaptation to allow females to locate them more easily (rarest species should show greatest perching site fidelity).
Method: trapping and/or hand-netting transects across ridges and rivers (two common perching microhabitats) to examine whether rarer species (in terms of point abundance and representation among sites) occur exclusively at a particular microhabitat.
Suggested location: eastern lowlands.
Background: few studies on mate-locating behaviour within butterfly faunas, particularly in the tropics.
References:
**Scott, J. A. (1970["1968"]). Hilltopping as a mating mechanism to aid the survival of low density species. Journal of Research on the Lepidoptera, 7(4): 191-204.
**Scott, J. A. (1973["1972"]). Mating of butterflies. Journal of Research on the Lepidoptera, 11(2): 99-127.
**Hall, J. P. W. (1999) A Revision of the Genus Theope: its Systematics and Biology (Lepidoptera: Riodinidae: Nymphidiini). Scientific Publishers, Gainesville. vii + 127pp.
**Callaghan, C. J. (1983). An study of isolating mechanisms among neotropical butterflies of the subfamily Riodininae. Journal of Research on the Lepidoptera, 21(3): 159-176.
**Alcock, J. (1983). Territoriality by hilltopping males of the great purple hairstreak, Atlides halesus (Lepidoptera, Lycaenidae): convergent evolution with a pompilid wasp. Behavioral Ecology and Sociobiology, 13: 57-62.
**Alcock, J. (1988). The mating systems of three territorial butterflies in Costa Rica. Journal of Research on the Lepidoptera, 26(1/4): 89-97.
**Lederhouse, R. C. (1982). Territorial defense and lek behaior of the black swallowtail butterfly, Papilio polyxenes. Behavioral Ecology and Sociobiology, 10: 109-118.
**Shields, O. (1968). Hilltopping. An ecological study of summit congregating behavior of butterflies on a southern California hill. Journal of Research on the Lepidoptera, 6(2): 69-178.
Relationship between lifespan and perching behaviour
Hypothesis: investment in territorial perching is correlated with shorter lifespans for species compared to those that patrol for mates.
Method: Select two groups of, ideally closely related, common species, one that shows obvious territorial perching behaviour and one that does not, and conduct MRR study to determine movement of males and estimates of lifespan.
Suggested location: eastern lowlands.
Background: yet to be investigated.
References:
**Alcock, J. (1987). Leks and hilltopping in insects. Journal of Natural History, 21: 319-328.
**Alcock, J. (1983). Territoriality by hilltopping males of the great purple hairstreak, Atlides halesus (Lepidoptera, Lycaenidae): convergent evolution with a pompilid wasp. Behavioral Ecology and Sociobiology, 13: 57-62.
**Lederhouse, R. C. (1982). Territorial defense and lek behaior of the black swallowtail butterfly, Papilio polyxenes. Behavioral Ecology and Sociobiology, 10: 109-118.
*Scott, J. A. (1973["1972"]). Mating of butterflies. Journal of Research on the Lepidoptera, 11(2): 99-127.
*Baker, R. R. (1983). Insect territoriality. Annual Review of Entomology, 28: 65-89.
Rutowksi, R. L. (1984). Sexual selection and the evolution of butterfly mating behaviour. Journal of Research on the Lepidoptera, 23(2): 125-142.
Other
Relationships between range-size and abundance
Hypothesis: abundance and range-size are positively correlated.
Method: measure local species abundance using hand-netting/trapping. Compare geographic range-size with number of sample sites at which species recorded and mean local abundance.
Suggested location: lowland forest.
Background: no data yet analysed for tropical butterflies.
References:
**Brown, J. H. (1984). On the relationship between abundance and distribution of species. American Naturalist, 124(2): 255-279.
**Brown, J. H., D. W. Mehlman, and G. C. Stevens (1995). Spatial variation in abundance. Ecology, 76(7): 2028-2043.
**Schoener, T. W. (1987). The geographical distribution of rarity. Oecologia, 74: 161-173.
**Quinn, R. M., K. J. Gaston, T. M. Blackburn, and B. C. Eversham (1997). Abundance-range size relationships of macrolepidoptera in Britain: The effects of taxonomy and life history variables. Ecological Entomology, 22(4): 453-461.
Gaston, K. J., T. M. Blackburn, and J. H. Lawton (1997). Interspecific abundance-range size relationships: an appraisal of mechanisms. Journal of Animal Ecology, 66(4): 579-601.
Gaston, K. J., T. M. Blackburn, and R. D. Gregory (1997). Interspecific abundance-range size relationships: range position and phylogeny. Ecography, 20: 390-399.
Flight height stratification in forest butterflies and morphology
Hypothesis: butterfly flight height is correlated with morphology (expect that species that occur in the canopy, at higher ambient temperatures, fly faster and have a lower wing area to thoracic mass ratio).
Method: set replicate canopy and understorey traps to measure preferred flight height, and measure thoracic (and total) mass for a small sample of collected specimens.
Suggested location: eastern lowlands.
Background: effect of habitat on morphology and physiology poorly understood.
References:
**Hall, J. P. W., and Willmott, K. R. (1999). Patterns of feeding behavior in adult male riodinid butterflies and their relationship to morphology and ecology. Biological Journal of the Linnean Society, 69: 1-23.
**Srygley, B., and P. Chai (1990). Predation and the elevation of thoracic temperature in brightly colored Neotropical butterflies. American Naturalist, 135(6): 766-787.
**Chai, P., and B. Srygley (1990). Predation and the flight, morphology and temperature of neotropical rain-forest butterflies. American Naturalist, 135(6): 748-765.
*Scott, J. A. (1983["1982"]). Mate-locating behavior of western north American butterflies. II. New observations and morphological adaptations. Journal of Research on the Lepidoptera, 21(3): 177-187.
Association of mimetic rings with microhabitat disturbance and/or flight height
Hypothesis: a diversity in mimicry rings is maintained by different microhabitat associations.
Method: hand-netting transects to document flight height and abundance of different mimicry rings in forest subjected to varying degrees of disturbance.
Suggested location: any forest east of the Andes from sea-level to 2000m.
Background: different mimicry rings may be linked to different adult or larval feeding behaviour, defence strategies or sexual signals; switching between rings is likely to lead to speciation. A diversity of mimicry rings may be maintained by different processes, depending on the taxon. Understanding what causes diversity in mimicry rings may help understand how mimetic groups have diversified.
References:
**Willmott, K. R., and J. Mallet (2004). Correlations between adult mimicry and larval hostplants in ithomiine butterflies. Proceedings of the Royal Society of London B (Suppl.).
**DeVries, P. J., and R. Lande (1999). Associations of co-mimetic ithomiine butterflies on small spatial and temporal scales in a neotropical rainforest. Biological Journal of the Linnean Society, 67: 73-85.
**Beccaloni, G. W. (1997). Vertical stratification of ithomiine butterfly (Nymphalidae: Ithomiinae) mimicry complexes: the relationship between adult flight height and larval host-plant height. Biological Journal of the Linnean Society, 62: 313-341.
*Mallet, J. L. B., and M. Joron (1999). Evolution of diversity in warning color and mimicry: polymorphisms, shifting balance and speciation. Annual Review of Ecology and Systematics, 30: 201-233.
Mallet, J., and L. E. Gilbert (1995). Why are there so many mimicry rings? Correlations between habitat, behaviour and mimicry in Heliconius butterflies. Biological Journal of the Linnean Society, 55:159-80.
General references
Familiarity with butterfly taxonomic groups occurring in region:
d'Abrera, B. (1981). Butterflies of the Neotropical Region, Part I. Papilionidae & Pieridae. East Melbourne, Lansdowne Editions. 172 pp.
d'Abrera, B. (1984). Butterflies of the Neotropical Region. Part II. Danaidae, Ithomiidae, Heliconidae & Morphidae. Ferny Creek, Hill House. Pp. 173-384.
d'Abrera, B. (1987a). Butterflies of the Neotropical Region. Part III. Brassolidae, Acraeidae & Nymphalidae (partim). Victoria, Black Rock, Hill House. Pp. 377-525.
d'Abrera, B. (1987b). Butterflies of the Neotropical Region. Part IV. Nymphalidae (partim). Victoria, Black Rock, Hill House. Pp. 527-678.
d'Abrera, B. (1988). Butterflies of the Neotropical Region. Part V. Nymphalidae (conc.) & Satyridae. Victoria, Black Rock, Hill House. Pp. 679-877.
d'Abrera, B. (1994). Butterflies of the Neotropical Region. Part VI. Riodinidae. Victoria, Black Rock, Hill House. Pp. 880-1096.
d'Abrera, B. (1995). Butterflies of the Neotropical Region. Part VII. Lycaenidae. Victoria, Black Rock, Hill House. Pp. 1098-1270.
DeVries, P. J. (1987) The Butterflies of Costa Rica and their Natural History. Papilionidae, Pieridae, Nymphalidae. Princeton: Princeton University Press. xxii+327 pp.
DeVries, P. J. (1997) The Butterflies of Costa Rica and their Natural History. Volume II. Riodinidae. Princeton: Princeton University Press. xxv + 288 pp.
Lewis, H. L. (1973). Butterflies of the World. Chicago: Follett. xvi + 312 pp.
Neild, A. F. E. N. (1996). The Butterflies of Venezuela. Part 1: Nymphalidae I (Limenitidinae, Apaturinae, Charaxinae). A comprehensive guide to the identification of adult Nymphalidae, Papilionidae and Pieridae. London: Meridian Publications. 144 pp.
Species richness on islands:
MacArthur, R. H., and E. O. Wilson (1967). The Theory of Island Biogeography. New Jersey: Princeton University Press.
Butterfly conservation:
Collins, N. M., and M. G. Morris (1985). Threatened Swallowtail Butterflies of the World. The IUCN Red Data Book. Gland: IUCN. vii+401 pp.
Collins, N. M., and J. A. Thomas (Eds.) (1999). The Conservation of Insects and their Habitats. London: Academic Press. Pp. 349-404.
New, T. R., R. M. Pyle, J. A. Thomas, C. D. Thomas, and P. C. Hammond (1995). Butterfly conservation management. Annual Review of Entomology, 40: 57-83.
Samways, M. J. (1994). Insect Conservation Biology. London: Chapman & Hall.
Sampling techniques:
Pollard, E., and T. J. Yates (1993). Monitoring Butterflies for Ecology and Conservation. London: Chapman & Hall.
Pullin, A. S. (ed.) (1995). Ecology and Conservation of Butterflies. London: Chapman & Hall.
DeVries, P. J., D. Murray, and R. Lande (1997). Species diversity in vertical, horizontal, and temporal dimensions of a fruit-feeding butterfly community in an Ecuadorian rainforest. Biological Journal of the Linnean Society, 62: 313-341.
Sparrow, H. R., T. D. Sisk, P. R. Ehrlich, and D. D. Murphy (1994). Techniques and guidelines for monitoring neotropical butterflies. Conservation Biology, 8(3): 800-809.
DeVries, P. J. (1988). Stratification of fruit-feeding nymphalid butterflies in a Costa Rican rainforest. Journal of Research on the Lepidoptera, 26(1-4): 98-108.
DeVries, P. J., and T. R. Walla (1999). Species diversity in spatial and temporal dimensions of fruit-feeding butterflies from two Ecuadorian rainforests. Biological Journal of the Linnean Society, 68: 333-353.
Soberón M., J., & J. Llorente (1993). The use of species accumulation functions for the prediction of species richness. Conservation Biology, 7(3): 480-488.








